News
What is a Quality Minute?
Posted by Cathy Wylie on
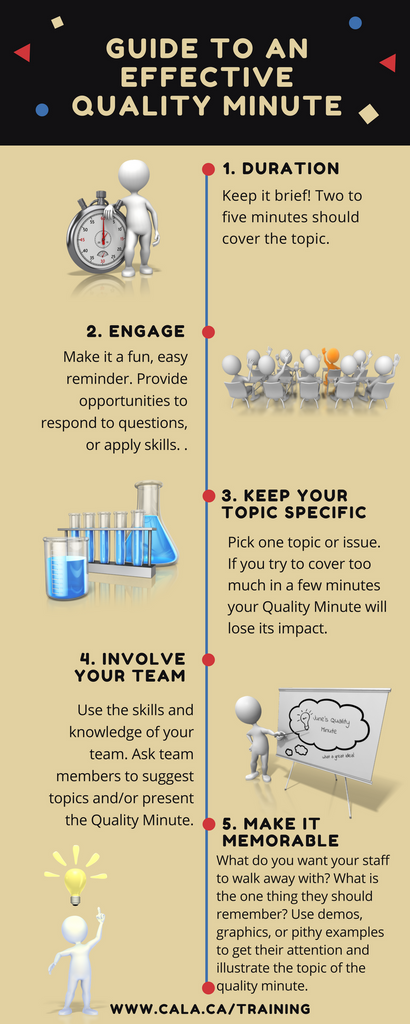
A Quality Minute is a concise talk about a specific quality system topic that is relevant to the work being done in your laboratory. It is usually included in a team meeting. The talks can be done in various ways, but are typically a short one to five minute discussions on a quality-related topic. For example: What details should be included in a Corrective Action Report (CAR)? How to store samples in the refrigerator Impact of failing to isolate/tag defective equipment What is the purpose of the equipment maintenance log? Quality Minutes serve as reminders about the quality system. They...
Use it or lose it - How to maximize your learning
Posted by Cathy Wylie on
After investing time, energy and money in training, how do you ensure you get the most out of your investment? The simple answer is to use what you have learned as soon as possible. Research shows that people retain only a small fraction of what they learn, and without some intervention, very little of what they learn gets transferred back to the workplace. Why is this the case? Your brain needs to work to encode, store and retrieve information. Encoding is how we take in new information. When you attend training, your brain works to relate the new information to...
- 0 comment
- Tags: Maximizing Learning
Do you know the difference between method "verification" and method "validation"?
Posted by Cathy Wylie on
ISO/IEC 17025:2005, Section 5.4.5 requires that methods are validated, and “…that the validation shall be as extensive as is necessary to meet the needs of the given application or field of application”. Further, the standard requires that the laboratory record the validation results, the procedure used for the validation, and a statement as to whether the method is fit for the intended use. The list of methods that require validation include non-standard methods, laboratory-designed/developed methods, standard methods used outside their intended scope, and amplifications and modifications of standard methods. But, what about “standard methods”? The requirements for “standard methods” can...
Funding available to train your laboratory staff!
Posted by Cathy Wylie on
The Canada Job Grant provides funds to help employers train new or existing employees. In most provinces, the Canada Job Grant will provide up to two-thirds of the cost of your training to a maximum of $10,000 per employee. Small businesses with 50 or fewer employees may be eligible to count wages as part of their employer contribution. The funds support skill development to qualify learners for new roles or increased responsibilities. The funds can be used towards CALA training courses. For example, you can train new internal auditors, train laboratory staff on validating methods and calculating uncertainty, or train...
Is your internal audit digging deep enough?
Posted by Cathy Wylie on
Internal Audits are a valuable tool in the laboratory. However, many audits simply skim the surface of the quality system and don’t dig in deeply enough to get the full benefit of the time spent on audits. Check out this list of audit best practices to help improve the quality of your audits.
- 0 comment
- Tags: Internal Audits